Wednesday 31 July 2013
Thursday 25 July 2013
Electrochemical energy storage and conversion
Electrochemical energy storage and conversion
The ideal and the real
For portable and transportation applications especially, a battery or fuel cell should store (and be able to deliver) the maximum amount of energy at the desired rate (power level) from a device that has the smallest possible weight and volume. The following parameters are commonly used to express these attributes:
Storage capacity or charge density, coulombs/liter or coulombs/kg;
Energy density, J/kg or watt-hour/lb
Power density, watts/kg
Voltage efficiency, ratio of output voltage to E°
Lifetime: shelf-life (resistance to self-discharge) or charge/recharge cycles
Physical limitations of battery performance
The most important of these are:
Effective surface area of the electrode. A 1-cm2 sheet of polished metal presents far less active surface than does one that contains numerous surface projections or pores. All useful batteries and fuel cells employ highly porous electrodes. Recent advances in nanotechnology are likely to greatly improve this parameter.
Current density of electrode surface. Expressed in amperes m–2, this is essentially a measure of the catalytic ability of the electrode, that is, its ability to reduce the activation energy of the electron transfer process.
Rate at which electroactive components can be delivered to or depart from the active electrode surface. These processes are controlled by thermal diffusion and are inhibited by the very narrow pores that are needed to produce the large active surface area.
Side reactions and irreversible processes. The products of the discharge reaction may tend to react with the charge-storing components. Thermal diffusion can also cause self-discharge, limiting the shelf life of the battery. Recharging of some storage batteries may lead to formation of less active modifications of solid phases, thus reducing the number of charge/discharge cycles possible.
Clearly, these are all primarily kinetic and mechanistic factors which require a great deal of experimentation to understand and optimize.

A battery is a galvanic cell in which some of the free energy change associated with a spontaneous electron-transfer reaction is captured in the form of electrical energy.
A secondary or storage battery is one in which the electron-transfer reaction can be reversed by applying a charging current from an external source.
A fuel cell is a special type of battery in which the reactants are supplied from an external source as power is produced. In most practical fuel cells, H+ ions are produced at the anode (either from H2 or a hydrocarbon) and oxygen from the air is reduced to H2O at the cathode.
The cathodic reduction of O2 is kinetically limited, necessitating the use of electrode surfaces having high catalytic activity.
The electrodes in batteries must have very high effective surface areas, and thus be highly porous. This requirement may conflict with the other important one of efficient diffusion of reactants and products in the narrow channels within the pores.
Batteries and fuel cells designed to power vehicles and portable devices need to have high charge-to-weight and charge-to volume ratios.
Net Calorific Value
Since the beginning of time, mankind had a good selection of fuels at his disposal and Table 1 provides the
The ideal and the real
For portable and transportation applications especially, a battery or fuel cell should store (and be able to deliver) the maximum amount of energy at the desired rate (power level) from a device that has the smallest possible weight and volume. The following parameters are commonly used to express these attributes:
Storage capacity or charge density, coulombs/liter or coulombs/kg;
Energy density, J/kg or watt-hour/lb
Power density, watts/kg
Voltage efficiency, ratio of output voltage to E°
Lifetime: shelf-life (resistance to self-discharge) or charge/recharge cycles
Physical limitations of battery performance
The most important of these are:
Effective surface area of the electrode. A 1-cm2 sheet of polished metal presents far less active surface than does one that contains numerous surface projections or pores. All useful batteries and fuel cells employ highly porous electrodes. Recent advances in nanotechnology are likely to greatly improve this parameter.
Current density of electrode surface. Expressed in amperes m–2, this is essentially a measure of the catalytic ability of the electrode, that is, its ability to reduce the activation energy of the electron transfer process.
Rate at which electroactive components can be delivered to or depart from the active electrode surface. These processes are controlled by thermal diffusion and are inhibited by the very narrow pores that are needed to produce the large active surface area.
Side reactions and irreversible processes. The products of the discharge reaction may tend to react with the charge-storing components. Thermal diffusion can also cause self-discharge, limiting the shelf life of the battery. Recharging of some storage batteries may lead to formation of less active modifications of solid phases, thus reducing the number of charge/discharge cycles possible.
Clearly, these are all primarily kinetic and mechanistic factors which require a great deal of experimentation to understand and optimize.

A battery is a galvanic cell in which some of the free energy change associated with a spontaneous electron-transfer reaction is captured in the form of electrical energy.
A secondary or storage battery is one in which the electron-transfer reaction can be reversed by applying a charging current from an external source.
A fuel cell is a special type of battery in which the reactants are supplied from an external source as power is produced. In most practical fuel cells, H+ ions are produced at the anode (either from H2 or a hydrocarbon) and oxygen from the air is reduced to H2O at the cathode.
The cathodic reduction of O2 is kinetically limited, necessitating the use of electrode surfaces having high catalytic activity.
The electrodes in batteries must have very high effective surface areas, and thus be highly porous. This requirement may conflict with the other important one of efficient diffusion of reactants and products in the narrow channels within the pores.
Batteries and fuel cells designed to power vehicles and portable devices need to have high charge-to-weight and charge-to volume ratios.
Net Calorific Value
Since the beginning of time, mankind had a good selection of fuels at his disposal and Table 1 provides the
Fuel
|
Energy by mass (Wh/kg)
|
Energy by volume (Wh/l)
|
Hydrogen (350 bar)*
|
39,300
|
750
|
Liquid hydrogen*
|
39,000
|
2,600
|
Propane
|
13,900
|
6,600
|
Butane
|
13,600
|
7,800
|
Diesel fuel
|
12,700
|
10,700
|
Gasoline
|
12,200
|
9,700
|
Natural gas(250 bar)
|
12,100
|
3,100
|
Body fat
|
10,500
|
9,700
|
Ethanol
|
7,850
|
6,100
|
Black coal(solid)
|
6,600
|
9,400
|
Methanol
|
6,400
|
4,600
|
Wood(average)
|
2.300
|
540
|
Li-cobalt battery
|
150
|
330
|
Li-manganese
|
120
|
280
|
Flywheel
|
120
|
210
|
NiMH battery
|
90
|
180
|
Lead acid battery
|
40
|
64
|
Compressed air
|
34
|
17
|
Supercapacitor
|
5
|
7
|
Table 1: Energy densities of fossil fuel and electrochemical batteries.Fossil fuel carries roughly 100 times the energy per mass compared to Li-ion.
Complied from various sources. Values are approximate.
* Hydrogen has the highest energy to mass ratio (Wh/kg), but energy by volume (Wh/l) reveals a truer picture in terms of storage and delivery. Diesel has almost 14 times the specific energy of pure hydrogen by volume (750Wh/l at 350 bar or 5,000psi).
Posted by
vasulu.b
at
02:15
0
comments
![]()
Tuesday 2 July 2013
Gibbs free energy and helm holtz free energy concept

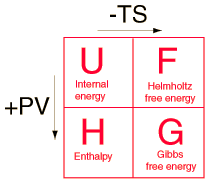
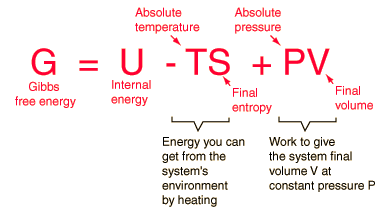

the Helmholtz free energy, an environment at constant temperature T will contribute an amount TS to the system, reducing the overall investment necessary for creating the system. This net energy contribution for a system created in environment temperature T from a negligible initial volume is the Gibbs free energy.
The Helmholtz free energy is of interest mainly to chemical engineers (whose industrial-scale processes are often confined to tanks and reactors of fixed volume) and some geochemists whose interest is centered on the chemistry that occurs deep within the earth's surface.
The free energy enables us to do this for changes that occur at a constant temperature and pressure (the Gibbs free energy) or constant temperature and volume (the Helmholtz free energy.)
Posted by
vasulu.b
at
00:08
0
comments
![]()
Subscribe to:
Posts (Atom)